Abstract
Background: In the era of treating childhood acute lymphoblastic leukemia (ALL) without cranial irradiation, optimization of central nervous system (CNS) active therapeutic agents is needed to prevent CNS relapse. Dexamethasone is an essential part of maintenance therapy in most current protocols for its excellent CNS penetration and anti-leukemic effect. The dose and duration of dexamethasone treatment for low-risk patients vary across trials. In our institution, we adopted the St Jude Total XV protocol with minor modifications since 2002. This treatment has resulted in 5-year OS and EFS rates of 96.4% and 94% respectively for low-risk patients. However, around 11% of patients developed serious viral infections, mainly disseminated varicella (6%), CMV retinitis (3.6%) and progressive multifocal encephalopathy (1.1%). The aim of this trial was to decrease the incidence of serious viral infections by decreasing the dose and the duration of dexamethasone treatment during maintenance therapy.
Patients and Methods: This is a prospective, non-randomized trial of all consecutive low-risk ALL patients aged 1-18 years treated at the Children's Cancer Center of Lebanon that included patients diagnosed after June 2013 as well as patients diagnosed earlier who had not reached week 100 of maintenance therapy by June 2013. Low-risk patients were defined elsewhere [Muwakkit et al, Am J Hematol. 2012 Jul;87(7):678-83].
For patients diagnosed after June 2013; dexamethasone dose was decreased by 25% (from 8 to 6 mg/m2/day for 5 days/week every 4 weeks) and dexamethasone pulses were stopped at week 69 instead of week 100 from start of maintenance therapy. For patients on therapy who had not reached week 69 of maintenance; dexamethasone pulses were stopped at week 69, and dexamethasone dose was decreased by 25% (from 8 to 6 mg/m2/day for 5 days/week every 4 weeks) starting June 2013. For patients who had completed 69 weeks but did not reach week 100 of maintenance by June 2013, dexamethasone pulses were stopped. The interval between dexamethasone pulses was 4 weeks for all patients, before and after the change.
The number of intrathecal chemotherapy injections was not changed.
For analysis, historical control patients who completed therapy before June 2013 and patients on therapy who completed more than half of their planned dexamethasone pulses (week 50) by June 2013 were assigned to group 1. Patients who were diagnosed after June 2013 and those who had received less than half of their planned dexamethasone pulses (week 50) by June 2013 were assigned to group 2.
The trial was stopped in May 2017 due to the observed increased incidence of isolated CNS relapse in group 2.
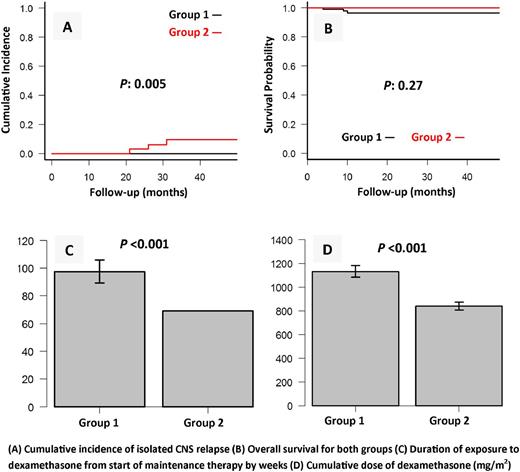
Results: The total number of patients was 84 in group 1 and 33 in group 2. There was no significant difference in patient characteristics between the two groups. After a median follow-up of 88 months (range: 24-177), the 4-year cumulative incidence of isolated CNS relapse increased from 0% in group 1 to 9.5% (n=3) (95% CI: 0-19.2%) in group 2 (P : 0.005). The mean cumulative dose of dexamethasone was 1129 mg/m2 (SD 49 mg/m2) for group 1 and 838 mg/m2 (SD 31 mg/m2) for group 2 (P : <0.001). The mean total duration of dexamethasone pulses from start of maintenance treatment was 97 weeks (SD 8 weeks) for group 1 and 69 weeks (SD 0 weeks) for group 2 (P <0.001). The cumulative dose of dexamethasone received by patients with isolated CNS relapse was 824, 876 and 930 mg/m2. The isolated CNS relapses occurred at weeks 74, 95 and 116 of maintenance therapy. None of the patients with isolated CNS relapse had an initial traumatic lumbar puncture. None of group 2 patients developed disseminated varicella, CMV retinitis, or progressive multifocal encephalopathy. None of the patients in both groups developed bone marrow relapse within 4 years of diagnosis. The 4-year cumulative incidence of infection-related mortality was 3.5% (95% CI: 0-7.4%) for group 1 and 0% for group 2 (P : 0.27). The 4-year OS rate was 96.4% (95% CI: 89.3-98.8%) for group 1 and 100% for group 2 (P : 0.27). The 4-year EFS rate was 96.4% (95% CI: 89.3-98.8%) for group 1 and 90.5% (95% CI: 73.2-96.8%) for group 2 (P : 0.24).
Conclusions: Decreasing dose and duration of dexamethasone therapy for low-risk childhood ALL patients aiming to avoid serious viral infections led to significant increase in isolated CNS relapse. Further studies are needed to identify the optimal dose and duration of dexamethasone therapy for these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.